TUAN NGUYEN DUC1,2, ANCHAYA MONGKOLCHAIYAPHRUEK3, VARIT SRILAONG4, SASITORN TONGCHITPAKDEE1,5,6,*
1Department of Food Science and Technology, Faculty of Agro-Industry, Kasetsart University, 50 NgamWongWan Road, Chatuchak, Bangkok 10900, Thailand
2Department of Postharvest Technology, Faculty of Biotechnology and Food Technology, Thai Nguyen University of Agriculture and Forestry, Thai Nguyen, Vietnam
3Department of Horticulture, Faculty of Agriculture, Kasetsart University, Bangkok 10900, Thailand
4Postharvest Technology Program, School of Bioresources and Technology, King Mongkut’s University of Technology Thonburi, Bangkok 10140, Thailand
5Postharvest Technology Innovation Center, Commission on Higher Education, Bangkok 10400, Thailand
6Center for Advanced Studies for Agriculture and Food, KU Institute for Advanced Studies, Kasetsart University, Bangkok 10900, Thailand
*Corresponding author: sasitorn.ch@ku.th
Dragon fruit (Hylocereus undatus) is one of the important tropical fruits in many countries such as Vietnam, Thailand, and Malaysia. Postharvest life of dragon fruits is usually limited by yellowing and bract wilting. Exposure to ethylene during mix load transportation may hasten senescence of dragon fruits. The objective of this research was to study the effect of ethylene on the quality and enzyme activity of dragon fruit. Dragon fruits harvested at 30-34 days after anthesis were dipped in ethephon at concentrations of 0, 100, 200, 400 and 800 ppm for 2 min, and stored at room temperature (27±2oC) for 8 days. Color (L*, a*, and b*), texture (compression test), total soluble solids (refractometer), respiration rate and ethylene production (gas chromatograph TCD and FID, respectively), peroxidase (POD), and phenylalanine ammonia-lyase (PAL) activities, phenolic content, and reducing sugar content (spectrophotometer) of samples were determined during storage. On day 4 and day 6 of storage, total color difference (rE) of fruit peel increased as ethylene concentration. The results showed that firmness of fruit pulp significantly decreased as the concentration of ethylene increased. Generally, respiration rate of dragon fruits increased with storage time and decreased after 6 days of storage. The results also showed that ethylene treatment had no significant effect on ethylene production of dragon fruits (p≥0.05). POD and PAL activities of dragon fruits in all treatments were significantly increased after storage. Ethylene treatment (100-800 ppm) had significant effect on color, firmness, respiration rate, and enzyme activity when compared to control.
Keywords: Dragon fruit, Ethylene, Quality, Enzyme activity, Postharvest life
1. INTRODUCTION
Dragon fruit is one of the important tropical fruits in many countries such as Vietnam, Thailand, and Malaysia. The dragon fruit is the good nutritious fruit. Several studies showed that dragon fruit is a good source of minerals, glucose, fructose, dietary fiber, and vitamins (Berbeu, 1990; Wu and Chen, 1997). Ethylene has been used commercially to hasten ripening and promote a uniform appearance of fruit (Reid, 1992). However, ethylene is not always beneficial for postharvest shelf life (Fresh, 2000). Exposure to ethylene could shorten shelf life of produce due to hasten senescence, loss of chlorophyll and increased susceptibility of product to microbial (Fresh, 2000). For dragon fruits, the main postharvest problem is senescence and yellowing in bract. Therefore, the objective of this research was to study the effect of ethylene concentrations on the quality and enzyme activity of dragon fruit during postharvest period.
2. MATERIALS AND METHODS
2.1. Raw material
Dragon fruits were harvested at 30-34 days after anthesis from the grower in Pratumthani, Thailand. The fruits were selected for uniformity of size and no mechanical damage condition. The prepared fruits were separated into 5 treatments as follows: 1) Distilled water (control), 2) 100 ppm of ethephon solution, 3) 200 ppm of ethephon, 4) 400 ppm of ethephon, and 5) 800 ppm of ethephon. 45 dragon fruits per each treatment were put into ethephon solution in 2 minutes, stored at room temperature (27±2oC), and observed at 0, 2, 4, 6 and 8 days after storage.
2.2. Quality assessment
Changes in the color of peel were determined using an UltraScan Pro (Hunter Lab, Reston, VA, USA) by the method of Gui et al., 2006. The pulp firmness was evaluated with a Texture Analyzer (TA.XT. Plus, Stable Micro System, UK) (Zhou et al., 2007). The total phenolic content in dragon fruit was determined by the method of Joubert et al., 2008. Total soluble solids (TSS) were measured using a hand refractometer (Model PR-1, Japan). Reducing sugar content was assayed as described by Somogyi (1952). Respiration rate and ethylene production were measured with a gas chromatograph (GC, Model 6890 N, Agilent, USA with TCD, and FID, respectively). Peroxidase (POD) and phenylalanine ammonia-lyase (PAL) activities were detected as described by Liu et al. (2007), and the method of Zucker (1965), respectively.
The experiment was designed using a Completely Randomized Design (CRD). Each treatment was comprised of two replicates. Statistical comparisons were made by analysis of variance (ANOVA) followed by a Duncan multiple range test using SPSS 20.0 statistical software (SPSS Inc., Chicago, IL). For all statistics, p<0.05 was considered to be the statistically significant difference.
3. RESULTS AND DISCUSSIONS
On day 4 and day 6 of storage, total color difference (rE) of fruit peel increased as ethylene concentration (Fig. 1a). Similar results were shown in yellow pitahaya fruits by Deaquiz et al., 2014. Treatment with ethylene accelerated chlorophyll degradation and increased an appearance of yellow or orange color of carotenoid pigments (Saltveit, 1999). The firmness of fruit pulp significantly decreased as the concentration of ethylene increased (Fig. 1b). The firmness of the control fruit slightly declined, whereas the firmness of ethylene treated fruits had greater decreased from the initial date to day 6 of storage. Loss of firmness may be related to the action of hydrolase enzymes (polygalacturonase, pectin methyl esterase, pectate lyase) (Giovannoni, 2004; Goulao et al., 2007). Those enzymes have weakened the cell walls, reduced the tensile force, and accelerated the softening of the dragon fruit (Deaquiz et al., 2014).
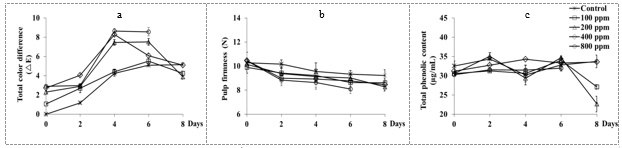
Figure 1 Total color difference (rE) of fruit peel (a), pulp texture (b) and total phenolic content (c) changes of dragon fruit stored at room temperature for 8 days.
Ethylene treatment had minimal changes total phenolic content, total soluble solids, and reducing sugar content of dragon fruits (Fig. 1c, 2a, and 2b, respectively)
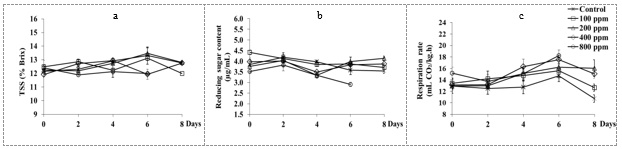
Figure 2 Total soluble solids (a), reducing sugar content (b) and respiration rate (c) changes of dragon fruit stored at room temperature for 8 days.
The respiration rates of dragon fruits tended to increase with storage time and then decreased after 6 days of storage at both treated and non-treated fruits (Fig. 2c). Respiration rate was higher in ethylene-treated samples when compared to control. In contrast, the ethylene production was not significantly affected by ethylene treatments (Fig. 3a). POD and PAL activities of dragon fruits in all treatments were significantly increased after storage (Fig. 3b, and 3c, respectively). However, fruits dipped in ethephon (100- 800 ppm) had no significant differences in POD activity when compared to control. PAL activity of fruits dipped in ethephon (100- 800 ppm) was significantly higher than those of untreated samples (p ≥0.05). The results were in agreement with those reported for Citrus fruit (Lafuente et al., 2004), in which ethylene increased PAL activity.

Figure 3 Ethylene production (a), peroxidase (b) and phenylalanine ammonia-lyase (c) activities changes of dragon fruit stored at room temperature for 8 days.
4. CONCLUSIONS
Ethylene treatment by dipping in ethephon (100-800 ppm) for 2 min had significant effects on color, firmness, respiration rate, and enzyme activity when compared to control. Minimal changes of total phenolic content, total soluble solids, and reducing sugar content were observed in ethylene-treated dragon fruits. Based on the results, dragon fruit should not be exposed to ethylene at a concentration of 100 ppm or more in order to maintain the quality and extend the shelf life of dragon fruit after harvesting.
ACKNOWLEDGEMENTS
The authors were gratefully acknowledged by Department of Food Science and Technology, Faculty of Agro-Industry, Kasetsart University for scholarship and their help during the research. This work was financially supported by Kasetsart University, Thailand.
REFERENCES
Berbeu G. 1990. The strawberry pear, a new tropical fruit. Fruits 45: 141-147.
Deaquiz Y.A, Alvarez-Herera J., Fischer G. 2014. Ethylene and 1-MCP affect the postharvest behaviour of yellow pitahaya fruits (Selenicereus megalanthus Haw.). Posthar. Biol. and Technol., Agron. Colomb. 32: 44-51.
Giovannoni J.J. 2004. Genetic regulation of fruit development and ripening. Plant Cell 16: 170-180.
Goulao L.F., Santos J., Sousa L.D., Oliveira C.M. 2007. Patterns of enzymatic activity of cell wall-modifying enzymes during growth and ripening of apples. Posthar. Biol. and Technol. 43: 307-318.
Gui F., Wu J., Chen F., Liao X., Hu X., Zhang Z., Wang Z. 2006. Change of polyphenol oxidase activity, color, and browning degree during storage of cloudy apple juice treated by supercritical carbon dioxide. Eur. Food Res. Technol. 223: 427-432.
Joubert E., Richards E.S., Van der Merwe J.D., De Beer D., Manley M., Gelderblom W.C.A. 2008. Effect of species variation and processing on phenolic composition and in vitro antioxidant activity of aqueous extracts of Cyclopia spp. (honeybush tea). J. Agric. and Food Chem. 56: 954-963.
Lafuente M.T., Sala J.M., and Zacarias L. 2004. Active oxygen detoxifying enzymes and phenylalanine ammonia-lyase in the ethylene-induced chilling tolerance in Citrus fruit. J. Afric. Food Chem. 52: 3606-3611.
Liu J., Tian S., Meng X., Xu Y. 2007. Effects of chitosan on control of postharvest diseases and physiological responses of tomato fruit. Posthar. Biol. and Technol. 44: 300-306.
Fresh O. 2000. The Fruit, Vegetable and Fresh Produce Storage Expert System. CSIRO Publishing, Collingwood Vic. Australia.
Reid M. 1992. Ethylene in Postharvest Technology, pp. 97-108. In "Postharvest Technology of Horticultural Crops". University of California.
Saltveit M.E. 1999. Effect of ethylene on quality of fresh fruits and vegetables. Posthar. Biol. and Technol. 15: 279-292.
Somogyi M. 1952. Notes on sugar determination. J. Biol. Chem. 195: 19-23.
Wu M.C., Chen S.C. 1997. Variation of sugar contents in various part of pitaya fruit. Proc. FI. State Hortic. Soc. 110: 225-227.
Zhou H., Forest M.G., Wang Q. 2007. Anchoring induced texture and shear banding of nematic polymers in shear cells. Disc. Contin. Dyn. Syst. Ser. B 8: 707-733.
Zucker M. 1965. Induction of phenylalanine deaminase by light and its relation to chlorogenic acid synthesis in potato tuber tissue. Plant Physiol. 40: 779-784.

| Đang online | 1057 |
| Hôm nay | 2174 |
| Hôm qua | 1533 |
| Tuần này | 19479 |
| Tuần trước | 33520 |
| Tháng này | 4478354 |
| Tháng trước | 3517132 |
| Tất cả | 51756572 |
Lượt truy cập: 51756586
Đang online: 1055
Ngày hôm qua: 1533
Phụ trách kỹ thuật: 0987. 008. 333